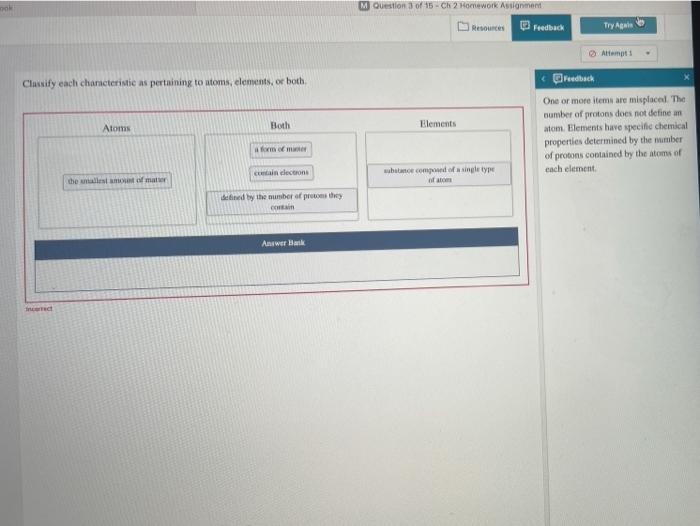
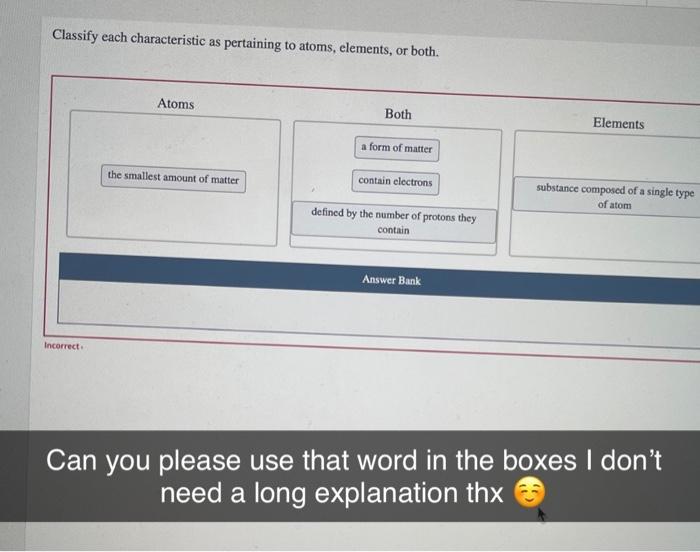
Classify each characteristic as pertaining to atoms elements or both. – In chemistry, understanding the fundamental building blocks of matter is crucial. This exploration delves into the classification of characteristics pertaining to atoms and elements, providing a clear distinction between these two essential concepts.
Atoms, the indivisible units of matter, possess unique properties that define their identity. Elements, on the other hand, represent collections of atoms with identical atomic numbers. By examining characteristics such as the number of protons, neutrons, and electrons, we can discern whether they pertain to atoms, elements, or both.
Classifying Characteristics of Matter: Classify Each Characteristic As Pertaining To Atoms Elements Or Both.
Matter, the physical substance that makes up the universe, can be classified into two fundamental entities: atoms and elements. Each of these entities possesses unique characteristics that distinguish them from one another. Understanding these characteristics is crucial for comprehending the structure and behavior of matter.
Classifying Characteristics, Classify each characteristic as pertaining to atoms elements or both.
The following characteristics can be classified as pertaining to either atoms or elements:
- Number of protons
- Number of neutrons
- Number of electrons
- Atomic mass
- Chemical symbol
- Reactivity
- Physical state
Differences Between Atoms and Elements
Definition of Atoms
Atoms are the fundamental building blocks of matter. They are composed of a nucleus, which contains protons and neutrons, and an electron cloud, which surrounds the nucleus.
Definition of Elements
Elements are pure substances that cannot be broken down into simpler substances by chemical means. Each element is characterized by its unique atomic number, which is equal to the number of protons in its nucleus.
Relationship Between Atoms and Elements
Atoms of the same element have the same atomic number but may have different numbers of neutrons, resulting in different isotopes of the element. Elements, on the other hand, are composed of atoms with the same atomic number.
Table Comparing Properties of Atoms and Elements
| Property | Atom | Element |
|---|---|---|
| Number of protons | Characteristic of an atom | Characteristic of an element |
| Number of neutrons | Characteristic of an atom | Varies among isotopes of an element |
| Number of electrons | Characteristic of an atom | Equal to the number of protons in an atom |
| Atomic mass | Characteristic of an atom | Average mass of all isotopes of an element |
| Chemical symbol | Represents an atom | Represents an element |
| Reactivity | Characteristic of an atom | Characteristic of an element |
| Physical state | Characteristic of an atom | Characteristic of an element |
Importance of Classification
Classifying characteristics as pertaining to atoms or elements helps us understand the structure of matter. By understanding the properties of atoms and elements, we can predict the behavior of matter and design materials with specific properties.
For example, the reactivity of an element is determined by the number of valence electrons in its atoms. This knowledge allows us to predict the chemical reactions that an element will undergo and to design materials with specific reactivity.
Question & Answer Hub
What is the difference between an atom and an element?
An atom is the smallest indivisible unit of matter, while an element is a collection of atoms with the same atomic number.
Which characteristics pertain to both atoms and elements?
Number of protons, number of electrons, and chemical symbol pertain to both atoms and elements.